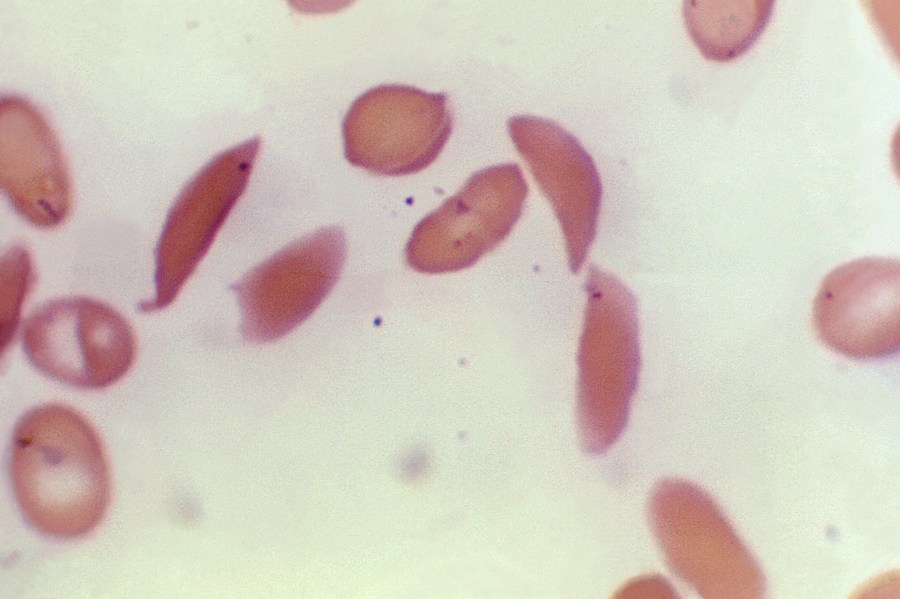
The Food and Drug Administration approved a new treatment for sickle cell disease on Friday. This is the first time that federal regulators have approved CRISPR gene-editing. The FDA approved two new treatments on Friday for sickle cell (SCD) disease: Casgevy, and Lyfgenia. Casgevy (also known as exacel) is developed using a…
The Food and Drug Administration ( FDA ) approved a novel treatment for sickle cell disease on Friday, making it the first time that federal regulators had given their blessing to CRISPR gene editing. Casgevy and Lyfgenia, two new treatments for sickle cell disease (SCD ), received FDA approval on Friday. Exa- cel, even known as casgevy, is created through a… The Food and Drug Administration ( FDA ) approved a novel treatment for sickle cell disease on Friday, making it the first time that federal regulators had given their blessing to CRISPR gene editing. Casgevy and Lyfgenia, two new treatments for sickle cell disease (SCD ), received FDA approval on Friday. Exa-cel, also known as ccasgevy, is created using…
The Food and Drug Administration approved a new treatment for sickle cell disease on Friday. This is the first time that federal regulators have approved CRISPR gene-editing. The FDA approved two new treatments on Friday for sickle cell (SCD) disease: Casgevy, and Lyfgenia. Casgevy (also known as exacel) is developed using a…