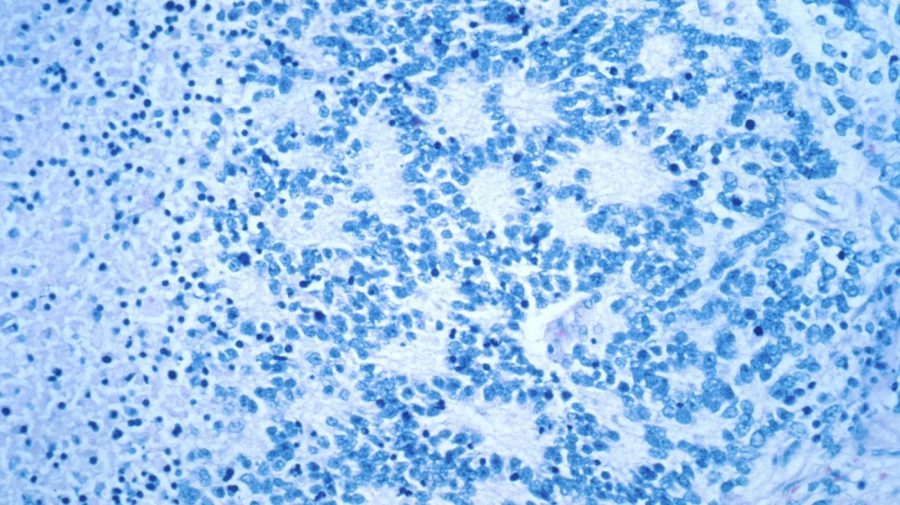
The Food and Drug Administration has required a boxed caution on all CAR-T cancer therapies. This follows a review that found the therapies can increase the risk of secondary cancers. In letters dated Jan. 19 to all six manufacturers of CAR-T cancer treatments currently available, the FDA stated that the prescribing…
Following a review of reports indicating that the therapies themselves may raise the risk for some secondary cancers, the Food and Drug Administration ( FDA ) is mandating that all current CAR-T cancer treatments be given boxed warnings. The FDA stated the prescribing information in letters dated January 19 to the manufacturers of all six CAR-T therapies that are currently on the market. Following a review of reports indicating that the therapies themselves may raise the risk for some secondary cancers, the Food and Drug Administration ( FDA ) is mandating that all current CAR-T cancer treatments be given boxed warnings. The FDA stated the prescribing information in letters to the manufacturers of all six already accessible CAR- T therapies dated January 19.
The Food and Drug Administration has required a boxed caution on all CAR-T cancer therapies. This follows a review that found the therapies can increase the risk of secondary cancers. In letters dated Jan. 19 to all six manufacturers of CAR-T cancer treatments currently available, the FDA stated that the prescribing…